The state of Louisiana filed a federal lawsuit against the U.S. Food and Drug Administration (FDA) for allowing pro-abortion doctors to mail the abortion pill into pro-life states with laws protecting the lives of preborn children.
Attorneys from Alliance Defending Freedom joined the lawsuit, as did Louisiana resident Rosalie Markenzich. In October 2023, Rosalie’s then-boyfriend obtained abortion drugs via mail from a doctor in California and pressured her to take them against her will.
“If the Biden FDA had not removed in-person dispensing, my then-boyfriend would not have been able to obtain abortion drugs and pressure me to take them against my will,” Rosalie said.
You can learn more about Rosalie’s story below:
The FDA places Risk Evaluation and Mitigation Strategy (REMS) requirements on only the most dangerous drugs which have “serious safety concerns.” Of the over 20,000 prescription drugs approved by the FDA, only a few dozen have REMS requirements. Mifepristone is one of them.
In 2023, the Biden administration modified its REMS for mifepristone (the abortion pill), permanently doing away with the “in-person dispensing requirement” for the drug. This change allowed women – and, apparently, their boyfriends – to begin accessing the drug through the mail rather than from a licensed physician in an office setting.
At the same time, the Biden FDA also created a new pharmacy certification process allowing retail pharmacies – like Walgreens and CVS – to sell the abortion pill.
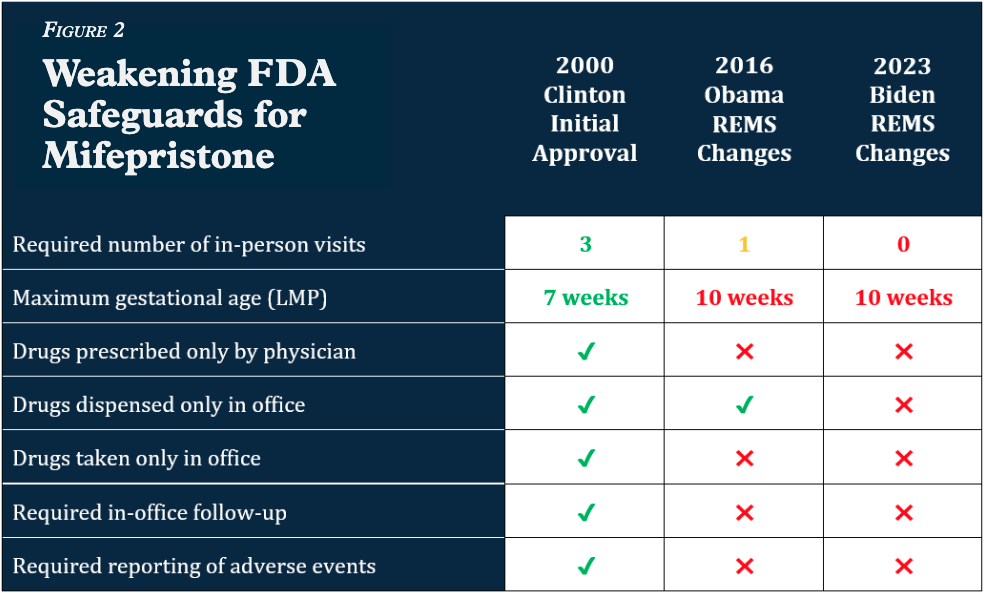
Photo Credit: Ethics and Public Policy Center
This change in the REMS requirements permits abortion drugs to flow from pro-abortion states into those with strong pro-life protections. This framework essentially nullifies the law in pro-life states, putting preborn babies nationwide at risk of abortion.
Louisiana’s lawsuit alleges the FDA’s 2023 REMS violates the Administrative Procedures Act (APA) and the Comstock Act, which prohibits the mailing of abortion drugs.
“The 2023 REMS – in its entirety – must be held unlawful, stayed, set aside, vacated, and preliminary and permanently enjoined under the [Administrative Procedures Act],” the lawsuit demands.
Louisiana Attorney General Liz Murrill issued a statement on the lawsuit, calling it an important case to protect the rights of women.
“Out-of-state abortion drug peddlers are violating the criminal laws of Louisiana and other states across the country that choose life,” Murrill said. “They aren’t providing healthcare, they’re drug dealers.”
The state attorney general added,
The state attorney general discussed the importance of the case in a video, which you can watch below:
The lawsuit comes at a crucial time as the FDA is reevaluating mifepristone’s safety profile, and considering whether the 2023 REMS adequately protects women from mifepristone’s many harms.
Let’s pray the lawsuit is successful in vindicating Rosalie’s rights and requiring the FDA to reinstate its earlier REMS requirements. We should also pray the FDA, even absent the lawsuit, will opt to raise the safety guardrails on mifepristone to protect preborn babies and their mothers from the dangerous abortion pill.
The case is Louisiana v. U.S. Food and Drug Administration.
At Focus on the Family, we have been working to turn the cultural tide and save mothers and babies from abortion for years. Since beginning the Option Ultrasound Program in 2004, Focus has helped save over half a million lives. Just $60 will help save a life through Option Ultrasound. Will you partner with us to save lives from abortion?
If you are experiencing an unexpected pregnancy and want to learn more about your options, you can visit My Choice Network.
Related articles and resources:
Counseling Consultation & Referrals
Dealing With Unplanned Pregnancy
The Abortion Pill: How Does It Work?
Become an Option Ultrasound Life Advocate
FDA Approves Generic Abortion Pill Despite Ongoing Safety Review
FDA Launches Review of Abortion Pill and the Harms it Causes Women
Photo from Alliance Defending Freedom.
The post Louisiana, ADF Challenge Biden-Era Abortion-By-Mail Scheme appeared first on Daily Citizen.